| Issue |
EPJ Photovolt.
Volume 3, 2012
Topical issue: Photovoltaic Technical Conference (PVTC 2011)
|
|
|---|---|---|
| Article Number | 35004 | |
| Number of page(s) | 13 | |
| DOI | https://doi.org/10.1051/epjpv/2012008 | |
| Published online | 14 August 2012 | |
https://doi.org/10.1051/epjpv/2012008
Kësterite thin films for photovoltaics : a review
1
EDF R&D, Institut de Recherche et Développement sur
l’Énergie Photovoltaïque (IRDEP), 6
quai Watier, 78401
Chatou,
France
2
CNRS, UMR 7174, 78401
Chatou,
France
3
Chimie ParisTech, 75005
Paris,
France
a
e-mail: delbos.sebastien@gmail.com
Received: 21 December 2011
Accepted: 21 May 2012
Published online: 14 August 2012
In the years to come, electricity production is bound to increase, and Cu2ZnSn(S, Se)4 (CZTS) compounds, due to their suitability to thin-film solar cells, could be a means to fulfill the demand. After explaining the reasons of the sudden interest of the CIGS scientific community for CZTS solar cells, this paper reviews recent papers published on the subject of kësterites-based solar cells. After a description of crystallographic and optoelectronic properties, including CZTS crystalline structure, defect formation and metal composition, this review paper focuses on CZTS synthesis processes and device properties. Synthesis strategies, including one- or two-step processes, deposition temperature, binary formation control via atmosphere control and their effect on device properties are discussed.
© Owned by the authors, published by EDP Sciences, 2012
 This is an Open Access article distributed under the terms of the Creative Commons Attribution-Noncommercial License 3.0, which permits unrestricted use, distribution, and reproduction in any noncommercial medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution-Noncommercial License 3.0, which permits unrestricted use, distribution, and reproduction in any noncommercial medium, provided the original work is properly cited.
1 Sustainability of photovoltaic industry
In the years to come, electricity production is bound to increase (from 17 PW h in 2009 to 28–34 PW h in 2035 [1]) and as a consequence the increased demand on fossil fuels as well as possible regulations on CO2 emissions are bound to increase the wholesale electricity costs. On the other hand, the price-experience factor of 22% that was observed for the last 4 decades should stay at the same level or slightly below [2, 3], leading to a decrease of photovoltaic (PV) electricity costs [2, 4]. A first change of paradigm is now happening, because in some places like South Italy and other sunny/high electricity price regions [5], the price of retail electricity is higher than the cost of PV electricity (grid parity). A second parity, the “fuel parity”, will happen when the cost of PV electricity is lower than the marginal costs of operating fossil fuel based power plants [6]. According to deployment scenarios, this fuel parity could happen between 2020 and 2040.
The massive deployment of PV foreseen in years 2020−2040 could lead to 1.5 to 2.5 TWp of worldwide installed PV capacity in 2030, with a demand of 20−150 GWp/y and around 30% of the market taken by thin films [2, 4].
The question of the sustainability of the production of 20−150 GWp/y of PV was raised by a few publications, and in particular the worldwide mining and refining capacity of a few critical elements could be the main problem of the photovoltaic industry in the years to come. Each PV technology has one or more limiting elements, such as silver for crystalline silicon, indium and gallium for CIGS, Tellurium for CdTe, Ruthenium for dye-sensitized solar cells, silver and indium for thin-film silicon, germanium, gold, indium for III-V PV [7, 8]. The chalcogenide technologies are particularly vulnerable to In, Ga, Te supply, which has been noted critical or near critical by the US Department of Energy [9] or the European Commission [10].
According to various authors, the production of PV modules could be limited below the demand reported earlier in this paper, as summarized in Table 1. Crystalline silicon PV seems suitable for supplying the demand, but one should keep in mind that the basic assumption used for the third and fourth column of this table is that all worldwide production of silver is used for crystalline PV. The fifth column shows the forecast 2030 demand for Ag, Te, In with respect to the 2008 production. It appears that the demand will grow enormously. If we focus on In, in 2030 the total demand (including PV and other uses) could be 3 to 7 times the 2008 production [10, 11], whereas, as In is a byproduct of Zn extraction, the elasticity of the production with respect to demand is low. One author suggests that CIGS and CdTe technologies will not be impeded by resources problems, but he does not take the increase of other sources of demand for In and Te into account [12].
The sustainability of PV production is therefore a real question, and the development of a new PV technology based on abundant and preferably non-toxic elements would alleviate the pressure on all PV technologies in term of resources.
2 Advantages of CZTS
Recently the CIGS scientific community started to work on Cu2ZnSn(S, Se)4, (CZTS), and the enthusiasm was kindled by the fast increase of record efficiency of CZTS-based solar cells, from the 2009 record of 6.7% [13] to 2011 record of 10.1% [14], as shown in Figure 1. CZTS has numerous advantages that could lead to its massive use as an abundant, non toxic, low cost absorber for thin-film photovoltaic solar cells :
-
it is a compound whose intrinsic point defects lead to p-type semiconductor behavior;
-
it has a direct bandgap and an absorption coefficient >104 cm-1, which is suitable for thin film photovoltaics applications [15, 16, 17];
-
its band gap has been predicted [18] to be 1.0 eV for Cu2ZnSnSe4 and 1.5 eV for Cu2ZnSnS4 and evidences of the bandgap tunability have been found via the variations of VOC [19], as well as direct measurements of the variation of the bandgap between 1.0 eV for Cu2ZnSnSe4 and 1.5 eV for Cu2ZnSnS4 [20, 21, 22, 23, 24]. The band gap of CZTS can also be tuned by incorporation of Ge, Ge-containing materials having smaller bandgap than their Ge-free counterparts [25]. This tunability is of particular interest for the manufacturing of absorbers with a band gap between 1.1 and 1.5 eV, which allow theoretical efficiencies higher than 30% [26];
-
its crystallographic structure can accept some shifts from the stoichiometric composition [27];
-
it includes Zn and Sn which are respectively produced in quantity 20 000 and 500 times bigger than In [28];
-
it is possible to make CZTS solar cells just by replacing CIGS by CZTS in CIGS solar cells, and such solar cells yielded efficiencies up to 10.1% [14, 29]. The knowledge gathered on back contact, buffer layers and window layer by CIGS scientists can therefore be used and adapted for CZTS solar cells. Nevertheless, for high efficiency solar cells the processes used for CIGS solar cells will need to be tuned. The buffer layer, in particular, will have to be tailored in order to adjust the lattice-matching, the valence and band offsets with CZTS;
-
the grain boundaries (GB) seem to have the same beneficial properties for CZTS as for CIGS, such as enhanced minority carrier collection taking place at the GB [30].
3 Crystallographic and optoelectronic properties
This paper is focused on synthesis processes of CZTS, and thus will only briefly address the crystallographic and optoelectronic properties. For more information on crystal and band structure and defects in kësterites, the reader can find a recent review dedicated to these topics here [31].
3.1 Crystalline structure
In the literature Cu2ZnSnS4 and Cu2ZnSnSe4 (as well as Cu2ZnGe(S, Se)4[32]) are described by the structural models of two natural minerals : stannite (space group I-42m) [33, 34, 35] and kësterite (space group I-4) [33]. These crystal structures are very close, in both structures the cations are located on tetrahedral sites but their distributions on planes perpendicular to the c-axis are not the same. In addition, the position of the chalcogen atom is slightly different in these structures [33, 34, 35].
-
kësterite : (Cu + Sn) / (Cu + Zn) / (Cu + Sn), chalcogen in position (x, y, z), Figure 2;
-
stannite : Cu / (Zn + Sn) / Cu, chalcogen in position (x, x, z).
In the rest of the paper, “kësterite” will be used for the crystallographic structure, and “kësterites” will be used for Cu2ZnSn(S, Se)4compounds that are crystallized in kësterite type structure. X will be used for the chalcogens S and Se when an effect or properties can apply to both of them (for example Cu2(S, Se) will be noted Cu2X).
Due to their structural similarities, kësterite and stannite are very difficult to distinguish by X-Ray Diffraction and Raman spectroscopy, and it is necessary to use neutron diffraction [36, 37] to tell them apart. According to ab initio calculi, the most stable crystalline structure is kësterite [38, 39, 40, 41, 42] and one study by ab initio calculation even suggests that all observations of stannite structure for CZTS compounds were due to partial disorder in the I-II (001) layer of the kësterite phase [32]. Recent neutron diffraction [37] and anomalous diffusion [43] characterizations show that CZTS crystallizes in the kësterite structure, and synchrotron X-Ray experiments show that this structure is dominant for temperature <876 °C (transition to a sphalerite cubic structure) [37].
The structural difference between Cu2ZnSnS4 and Cu2ZnSnSe4 is not clear. It seems that the Se compound has larger lattice parameters as well as higher electric conductivity than the S compound [20]. Concerning mechanical and thermo-physical properties of CZTS, no experimental data are available, but ab initio simulations were performed [44, 45].
Sulfur kësterites have XRD peaks very close to those of ZnS and Cu2SnS3[36], and Raman spectroscopy is necessary to tell them apart [46]. Nevertheless, it is not possible to have a quantitative determination of the secondary phases by Raman. For such purpose, XENAS has been used [47].
3.2 Intrinsic point defect formation
The kësterite structure allows for several types of point defects which make discrete energy levels appear, allowing p-type doping. As in the CIGS materials, the doping is obtained by stoichiometry variations and not by extrinsic doping. Nevertheless, according to ab initio calculi, the kësterite structure is less tolerant to these variations than the chalcopyrite structure [39, 48, 49, 50, 51]. According to point-defect measurements, the kësterites are susceptible to higher carrier recombination than chalcopyrite [52]. Measurements of the deep defects of CZTS are reported here [53].
Generally, the Sn-Se bond is much stronger than the Zn-Se and Cu-Se bond, leading to a high formation energy of VSn[51]. Contrary to CIGS, the accepting defect the most easily formed is not VCu but CuZn[39, 49, 54, 55]. Nevertheless some authors suggest the contrary [52, 56, 57]. ZnCu substitutions occur at the 2c site and CuZn at the 2d site [36, 58] (see Fig. 2). The accepting defects are easily formed, leading to difficult n-type doping [54]. The easy formation of CuZn accepting defects also leads to low charge separation, a drawback that could be overcome thanks to the type-II band alignment of the CZTS/CdS interface [49]. The most active recombination centers are expected to be CuSn (InCu for CIGS), and the lack of defect complex (2VCu + InCu for CIGS) is thought to lead to the higher defect concentration observed [59].
 |
Fig. 3 Composition map of the best CZTS solar cells. The diameter of the circles is proportional to the conversion efficiency. See Table 2 for the references. |
3.3 Metal composition
The single phase composition region is much narrower for CZTS than for CIGS. According to the Tallinn team, single phase CZTS monograin powders can be synthesized only from a precursor mixture comprising metal ratios of Cu/(Zn + Sn) = 0.92−0.95 and Zn/Sn = 1.0−1.03[60]. Nevertheless, a Rietveld refinement of anomalous dispersion measurements revealed a Cu/(Zn + Sn) = 0.97, and Zn/Sn = 1.42 composition for kësterites crystals [43], suggesting that the single phase composition region is not as narrow as the Tallinn team suggests.
Until now, only Zn-rich and Cu-poor materials gave high efficiency (see Fig. 3), as was already noted by the Nagaoka team [61]. In Zn-rich growth conditions, the kësterite structure is tolerant to stoichiometry deviations by zinc excess and copper lacking [61, 62, 63, 64], in the form of ZnCu and VCu vacancies [51, 65], but high Zn content also leads to structural disorder [66].
3.4 Bandgap measurement
Absorbance measurements are not suitable for extracting the bandgap values, because the absorption coefficient derived from spectrophotometric data on thin films is determined by defect absorption and by measurement accuracy limitations [66]. In particular, ZnSe [67] and Zn-caused disorders [66] lead to incorrect bandgap determination when it is determined from absorbance measurements. PL measurements now confirm that the bandgap of Cu2ZnSnSe4 is indeed around 1.0 eV at room temperature [68], whereas before that publication the measured band gap was around 1.5 eV. Suitable methods for measurement of CZTS bandgap are PL, TR-PL and EQE measurements.
Synthesis methods for CZTS solar cells.
Continued.
4 Synthesis strategies
 |
Fig. 4 SEM cross-section of CZTSe films grown at the same temperature and constant deposition fluxes, except Cu rates were adjusted to produce only (a) Cu-rich growth or (b) Cu-poor growth [59]. |
4.1 One-step or two step processes for thin films
For CIGS, the deposition techniques used to be classified as vacuum and non-vacuum techniques. For CZTS, the same approach was also used in a review [69]. Nevertheless, for CZTS, this classification is not accurate, because recent works showed that low-cost deposition does not mean low-cost solar modules [70], and because of the problematic of binaries control during CZTS synthesis (see next paragraph). We therefore decided to classify the processes into one-step or two-step processes :
-
Two-step processes, where the needed elements are first incor-porated during an ambient temperature process, followed byan annealing step. The chalcogen can be incorporated into theprecursor or during the annealing step. These processes allowthe use of fast and low-cost techniques for precursor deposi-tion. Until now, and contrary to the CIGS, two-steps processesyielded the best efficiencies (more than 10%) [14]. IBM workson three such processes, the hybrid solution-particle tech-nique [14], electrodeposition [71] and coevaporation [72].Other teams investigated numerous other deposition tech-niques, such as codeposition by evaporation, sputtering,OA-CVD (Open Atmosphere Chemical Vapor Deposition),nanoparticles, sol-gel (see Tab. 2).
-
One-step processes, where all the elements are incorporated simultaneously. This type of processes yields the better results for CIGS, but until now it was not the case for CZTS. The best efficiency until now is 9.15% by coevaporation [73]. Only a few groups published results for such processes, and very few had significant results in terms of solar cell efficiency : NREL [73], Tallinn University [74], HZB [75]. Other groups tried reactive sputtering and pulsed laser deposition (PLD) (see Tab. 2). The method of Tallinn University also introduces a huge difference in cell architecture, where 50 μm CZTS monograins are synthesized in molten KI, wrapped in the buffer layer and attached to the substrate by epoxy glue [76].
These processes are different, but the two types of processes (one-step and two-steps) reached high efficiencies (>9%). Until now, it does not seem that one or the other is intrinsically better, because, as we will see in the next paragraphs, the key point is temperature and atmosphere control during the one-step deposition or annealing step.
Other methods of synthesis that did not produce solar cells include : cosputtering of Cu, Sn, Zn followed by annealing in a hot tube in presence of H2S [77] , dip-coating in a methanol solution followed by annealing at 200 °C in air [78], CZTS nanoparticles in oleylamine [79] , chemical bath deposition [80] , sputtering of a sintered mix of CuSe, CuSe2 , ZnS, and SnS2[81].
4.2 CZTS formation
Similarly to CIGS, CZTS needs temperature between 500 and 600 °C to be synthesized. Nevertheless, the chemical reactions are not yet completely understood, even if a reaction path was proposed [82]. There are successive reactions taking place in the bulk of the layer between the elements leading to binaries, ternaries and finally the quaternary.
At room temperature, only metal binaries can form, for example Cu6Sn5[83], Cu5Zn8[84] , Cu3Sn and CuZn [85]. At higher temperature, between 200 and 450 °C, metal-chalcogenide binaries such as CuX, Cu2X, and SnX form [86, 87, 88], and for temperature >450 °C, CuxX binaries then react with Sn to form CuxSnyS [80]. At higher temperature (between 550 and 580 °C [71, 85, 86, 89]), and for longer annealing time (~8 h) or one-step deposition at 500 °C [73, 83] , ZnX reacts with Cu2SnX3 to form Cu2SnZnX4 according to the equation proposed by [82] :  (1)In order to control which reactions take place during the annealing step of a two-step process, it is necessary to closely monitor the temperature of the layer, because, for example, too fast an annealing can lead to liquid Sn bubbles formation, preventing the formation of large grains [90]. For processes using elemental sulfur or selenium, fast annealing (a few minutes) is possible, whereas for processes using H2S, which is less reactive than elemental sulfur, a long annealing time (2−3 h) is necessary for the binaries to react completely and to form large grains of CZTS [91, 92].
(1)In order to control which reactions take place during the annealing step of a two-step process, it is necessary to closely monitor the temperature of the layer, because, for example, too fast an annealing can lead to liquid Sn bubbles formation, preventing the formation of large grains [90]. For processes using elemental sulfur or selenium, fast annealing (a few minutes) is possible, whereas for processes using H2S, which is less reactive than elemental sulfur, a long annealing time (2−3 h) is necessary for the binaries to react completely and to form large grains of CZTS [91, 92].
For one-step processes, Cu-rich growth conditions are needed at the beginning of the reaction in order to foster growth of large grains [73, 93], as shown in Figure 4. Nevertheless, for both processes, the finished layer must be Zn-rich (see part 4.3), and in particular Cu2X-free [60] in order to get high photovoltaic efficiencies. If the layer is not Cu2X-free at the end of the synthesis process, the subsequent cyanide etching leads to possible voids and defects [89]. If Zn-rich growth conditions are used, ZnX formation is promoted [48, 49].
Therefore, for one-step processes it is necessary to adapt the rate of deposition of each element with respect to the reaction path, as well as control deposition temperature, because higher temperatures can also increase grain size [59]. For two-step processes it is necessary to closely monitor growth conditions, and in particular prevent Cu2X formation and increase ZnX reaction with Cu2SnS3.
4.3 Atmosphere control
Unfortunately, contrary to CIGS, the elements involved in CZTS synthesis are prone to evaporation and sublimation. Zn sublimates at 430 °C [94], SnSe at 350 °C [88], SnS at 370 °C and Sn evaporates at 460 °C [95, 96]. Moreover, high temperatures promote CZTS decomposition, according to the chemical equilibrium between CZTS and solid and gaseous binaries [97, 98]. 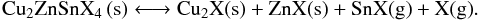 (2)If this equilibrium is displaced towards the right member, ZnX, SnX and X formation and losses will occur, leading to a Cu2X-rich layer, increasing layer resistance and preventing good photovoltaic efficiencies (see 5.6). It is therefore of the utmost importance to prevent CZTS decomposition as well as binaries losses. The key to that result is atmosphere control.
(2)If this equilibrium is displaced towards the right member, ZnX, SnX and X formation and losses will occur, leading to a Cu2X-rich layer, increasing layer resistance and preventing good photovoltaic efficiencies (see 5.6). It is therefore of the utmost importance to prevent CZTS decomposition as well as binaries losses. The key to that result is atmosphere control.
The equilibrium of equation (2) can be displaced towards CZTS by saturating the atmosphere in one of the right-side members of the equation [93, 97]. It is possible to counteract SnX evaporation by introducing gaseous SnX during the thermal treatment [98], or by increasing the partial pressure of the chalcogen [99], by performing the chalcogenation in a small closed volume [100], or by using a cap on the precursor layer during annealing [101]. If the annealing takes place in a H2 atmosphere, Zn loss can be prevented by using ZnS as a precursor [102]. Continuous evaporation of Zn towards the substrate prevents the decomposition of CZTS in a coevaporation process [73]. It also seems that the presence of MoX2 at the back contact pushes the reaction towards the right member [103].
If atmosphere control is not good, annealing time is the next best lever on CZTS decomposition, because if CZTS is synthesized and cooled rapidly, only a small quantity of binaries will be lost. One paper compared slow and fast annealing [104]. It concluded that fast annealing is better than slow annealing because many different secondary phases were found with slow annealing, without more explanation. Nevertheless, in this case slow annealing could be linked to a badly controlled reaction atmosphere, leading to CZTS decomposition into binary and ternary phases.
Concerning the key point of atmosphere control, strategies of synthesis should be slightly different for one-step or two-step processes. For two-step processes, there seems to be two stages during the annealing step : one short (a few minutes) step of CZTS formation, and one longer (up to a few hours) step of grain growth. The control of the atmosphere should be very accurate in the second step, and the reaction atmosphere could also be completely different from the first step [93]. Fortunately, it is quite easy and inexpensive to design annealing equipments that provide good static atmosphere control (that is to say, equipments where nothing can either go in or go out). Nevertheless, vapor injection is more tricky, as it requires carrier gas, pressure measurements, leading to an equipment resembling an evaporator.
For one-step synthesis, atmosphere control is not so easy, because reaction chambers are designed for controlling the deposition rate and not the partial pressure of each element. In order to prevent CZTS decomposition and binaries losses, Sn and chalcogen deposition rate should remain high during the cooling phase, in order to counteract Sn loss [73].
4.4 Composition gradient
The composition gradient either created by a variation of the rate of deposition of each element in a codeposition or by stacked elements, has an influence on CZTS film properties, because of Cu diffusion, Sn loss and chemical mechanisms of formation.
Concerning Cu, its diffusion can create voids at the back contact. Two-step processes, especially when the precursor layer is a codeposited metal layer or stacked metal layers with Cu as first layer, are subject to these voids [89, 103]. Secondary phases are observed, preventing the total formation of CZTS [105].
As explained in the previous paragraph (see Eq. (2)), Sn evaporates if the atmosphere is not well controlled. Nevertheless, the loss of Sn could also be prevented by having the Cu layer on top [63, 93].
Concerning the reaction mechanism, for stacked metals, it is beneficial to have Cu and Sn in close contact for the formation of large CZTS grains [106]. If they are separated by Zn or Zn(S, Se), the reaction between copper chalcogenides and tin chalcogenides, which is necessary to form Cu2SnX3[82], could be inhibited [107]. For stacked metals, Cu on top seems to foster formation of CuxS, which may result in larger grains and denser films [108]. For one-step processes, high Cu content at the beginning of the deposition promotes the formation of large grains [73].
Concerning the composition gradient,
-
for two-steps processes, the Cu must not be deposited first, andCu and Sn should be in close contact. The possible combinationsfor stacks are therefore Mo/Zn(X)/Cu/Sn or Mo/Zn(X)/Sn/Cu.The codeposition seems also possible, but with a Cu-poorcomposition near the back-contact;
-
for one-step processes, the layer must be Cu rich at the beginning of the deposition in order to promote the growth of large grains.
4.5 Sodium
Sodium seems to have the same effects on CZTS as on CIGS : it promotes the growth of larger grains, enhances conductivity and has a significant effect on film morphology, as was demonstrated by SLG / borosilicate substrate comparison and dipping in Na2S [109, 110]. Nevertheless, contrary to CIGS, Na was not detected by XPS at the surface, meaning that the Na diffusion in CZTS is smaller than in CIGS [111].
4.6 Device properties
CZTS devices are similar to CIGS devices. The architecture is usually SLG/Mo/CZTS/CdS/i-ZnO/ZnO :Al, as presented in Figure 5. Calculation showed that CdS has a suitable band offset with CZTS [112] and until now, the most efficient solar cells were obtained with a CdS buffer layer. Oxidation of the CZTS absorber by dipping in deionized water [13] or O2 annealing [73] before deposition of CdS seems to improve efficiencies. As for CIGS, reducing the time between absorber synthesis and CdS deposition to the minimum is of the utmost importance [106].
A few variations of the architecture are known, especially on the buffer layer. Pure ZnS does not seem to be a suitable buffer layer because of too high a band offset [112], but good results were obtained with Zn(S, O, OH) by Solar Frontier [113]. Nevertheless for such a buffer layer the optimal composition of the absorber layer seems to be less Zn-rich (Zn/Sn ~ 1) [113]. Other architectures were proposed : SLG/Mo/CZTS/a-Si/ITO [114], a superstrate architecture (glass/FTO/CZTS) [115, 116], another superstrate (glass/FTO/TiO2/In2S3/CZTS/Mo) [117], a flexible substrate (Al foil/Mo/CZTS/ZnS/i-ZnO/ITO/Al-Ni) [118].
 |
Fig. 5 Classical architecture for CZTS solar cells. |
The literature is still quite poor concerning device properties, because very few high-efficiency devices were synthesized yet. The IBM team started to investigate this field [14, 72, 119, 120] concluding that their devices were limited by the recombination at absorber/buffer interface, minority carrier lifetime and Schottky-type barrier in the back contact, which appears to be caused by secondary phases and/or MoSx interfacial layer between CZTS and Mo [121].
Concerning the absorber / buffer interface, it seems that the surface of the CZTS is Cu-poor, similarly to CIGS [111], or even Cu-free [122]. The KCN etching, which is also used in CIGS processes to remove binaries from the surface, seems to etch Cu preferentially and Sn in a lesser extent [93]. It seems also beneficial for removing Cu2X that increase the resistivity of the layer [123]. This causes a widening of the bandgap without type inversion, which could be an easy way to tailor bandgap alignments [124, 125]. It seems also that the electronic structure of a Sn-depleted surface is not favorable for the formation of well working p-n junction [74, 93]. The Conduction Band Offset, about 0.4−0.5 eV, is somewhat above the optimal range of 0−0.4 eV and may contribute to lower Jsc and fill factor in the CZTSSe devices [126]. Better lattice matching between absorber and buffer could limit recombination at the interface, and using different buffer with smaller lattice such as In2S3 or Zn(S, O, OH) could improve the efficiency of the devices [127, 128].
Concerning the back contact, ZnX was observed at the back contact [59, 106], as shown in Figure 6, and it seems that increasing the ZnX content strongly impairs efficiency [47]. It was nevertheless speculated that ZnS could be less detrimental than previously thought [72], acting as a simple “dark” material. Binaries losses that occur during annealing makes Cu2X to diffuse towards the rear of the cell, could lead to a Cu2X/MoX2 mix, leading to high resistance at the back contact.
Concerning minority carrier lifetime, it might not be such a problem, because as in CIGS and CdTe, grain boundaries collect minority carrier and provide a current pathway for them to reach the n-type CdS and ZnO layers [30] and for coevaporated absorbers, high minority carrier diffusion length was measured (several hundreds of nm [72]). Nevertheless, it seems that grain boundaries are Cu-rich, which could increase recombination rates and thus diminishing VOC[29]. Cu-rich absorbers were found to have lower VOC than Cu-poor ones [60], probably in relationship with Cu2X formation. Longer minority carrier lifetimes were measured in low band gap materials, such as CZTSe, than in high band gap devices [29]. The difference between Eg in eV and VOC in V, called VOC deficit, can therefore be decreased by diminishing the bang gap as well as tightly controlling the formation of Cu2X or its removal by KCN etching.
For high efficiency, it is important to focus both on absorber quality and on interfaces between the absorber and the back contact and buffer. The CuxX binaries control seems to be a key point for both absorber and interface quality. Fortunately, the CIGS community has a strong experience on such problems, and hopefully it will be possible to optimize these interfaces much faster than for the CIGS.
5 Conclusion
The problematics encountered by research teams working on CZTS-based solar cells were presented and discussed. The field of CZTS solar cells is young, and until now it is difficult to point to certainties. Nevertheless, it seems that these assumptions start to be viewed as “common knowledge” in the community :
-
CZTS has kësterite structure;
-
the bandgap is tunable between 1.0 eV (Cu2ZnSnSe4) and 1.5 eV (Cu2ZnSnS4);
-
the synthesis atmosphere must be controlled carefully;
-
binary formation control is a key point for high efficiency devices;
-
Zn-rich materials yield better results (when using a CdS buffer layer).
Nevertheless, there are numerous questions still, such as :
-
what are the crystalline defects, how are they formed; what roledo they play in the structure;
-
what is the reaction path to CZTS, how to control binaries formation;
-
what is the role of sodium during growth.
More and more scientists from the CIGS community work on CZTS solar cells. The problems encountered in CZTS solar cells are quite similar to the ones of CIGS solar cells, and similar methods can be used to solve them. There is no clear superiority of two-step processes over one-step processes, even if until now the best results were obtained with one-step processes. For both types of processes, a focus on binary control formation by atmosphere control and adaptation of interfaces to kësterites materials are the key points for increasing photovoltaic efficiencies for both types of processes.
Acknowledgments
The author is thankful for financial support of the French Agence Nationale de la Recherche (ANR) under grant NovACEZ (ANR-10-HABISOL-008).
Références
- International Energy Agency, World Energy Outlook 2011 (2011) [Google Scholar]
- Greenpeace and the European Photovoltaic Industry Association, Solar Generation VI (2011) [Google Scholar]
- European Climate Foundation, Roadmap 2050, a practical guide to a prosperous, low-carbon Europe (2010) [Google Scholar]
- International Energy Agency, Technology Roadmap, Solar photovoltaic energy (IEA, 2009) [Google Scholar]
- A. Zaman, S. Lockman, Solar industry growth : you ain’t seen nothing yet. The grid parity decade (Piper Jaffray Investment Research, 2011) [Google Scholar]
- C. Breyer, M. Görig, J. Schmid, Fuel-parity : impact of photovoltaics on global fossil fuel fired power plant business, presented at the 26, Symposium Photovoltaische Solarenergie, Bad Staffelstein (2011) [Google Scholar]
- C.S. Tao, J. Jiang, M. Tao, Solar Energy Mater. Solar Cells 95, 3176 (2011) [CrossRef] [Google Scholar]
- A. Feltrin, A. Freundlich, Renew. Energy 33, 180 (2008) [CrossRef] [Google Scholar]
- U.S. Department of Energy, Critical Materials Strategy (2010) [Google Scholar]
- European Commission, Critical Raw Materials for the EU (2010) [Google Scholar]
- A. Zuser, H. Rechberger, Resour. Conserv. Recycl. 56, 56 (2011) [Google Scholar]
- V. Fthenakis, Renew. Sust. Energy Rev. 13, 2746 (2009) [Google Scholar]
- H. Katagiri, K. Jimbo, W.S. Maw, K. Oishi, M. Yamazaki, H. Araki, A. Takeuchi, Thin Solid Films 517, 2455 (2009) [CrossRef] [Google Scholar]
- D.A.R. Barkhouse, O. Gunawan, T. Gokmen, T.K. Todorov, D.B. Mitzi, Prog. Photovol. Res. Appl. 20, 6 (2012) [Google Scholar]
- K. Ito, T. Nakazawa, Jpn J Appl. Phys. 27, 2094 (1988) [Google Scholar]
- C.P. Chan, H. Lam, C. Surya, Solar Energy Mater. Solar Cells 94, 207 (2010) [CrossRef] [Google Scholar]
- W. Xinkun, L. Wei, C. Shuying, L. Yunfeng, J. Hongjie, J. Semiconductors 33, 022002 (2012) [CrossRef] [Google Scholar]
- S. Chen, X.G. Gong, A. Walsh, S.H. Wei, Appl. Phys. Lett. 94, 041903 (2009) [CrossRef] [Google Scholar]
- K. Timmo, M. Altosaar, J. Raudoja, K. Muska, M. Pilvet, M. Kauk, T. Varema, M. Danilson, O. Volobujeva, E. Mellikov, Solar Energy Mater. Solar Cells 94 1889 (2010) [CrossRef] [Google Scholar]
- J. He, L. Sun, S. Chen, Y. Chen, P. Yang, J. Chu, J. Alloys Compd. 511, 129 (2012) [CrossRef] [Google Scholar]
- M. Moynihan, G. Zoppi, R. Miles, I. Forbes, Investigating synthesis of Cu2ZnSn(Se1−x, Sx)4 for values of 0 ≤ x ≤ 1 by S for Se substitution and direct sulphidisation of metallic precursors, presented at the 26th European Photovoltaic Solar Energy Conference and Exhibition, Hamburg (2011) [Google Scholar]
- J. He, L. Sun, N. Ding, H. Kong, S. Zuo, S. Chen, Y. Chen, P. Yang, J. Chu, J. Alloys Compd. 529, 34 (2012) [CrossRef] [Google Scholar]
- E. Mellikov, D. Meissner, M. Altosaar, M. Kauk, J. Krustok, A. Öpik, O. Volobujeva, J. Iljina, K. Timmo, I. Klavina, J. Raudoja, M. Grossberg, T. Varema, K. Muska, M. Ganchev, S. Bereznev, M. Danilson, AMR 222, 8 (2011) [CrossRef] [Google Scholar]
- S. Levcenco, D. Dumcenco, Y.P. Wang, Y.S. Huang, C.H. Ho, E. Arushanov, V. Tezlevan, K.K. Tiong, Opt. Mater. 34, 1362 (2012) [CrossRef] [Google Scholar]
- G.M. Ford, Q. Guo, R. Agrawal, H.W. Hillhouse, Chem. Mater. 23, 2626 (2011) [CrossRef] [Google Scholar]
- W. Shockley, H.J. Queisser, J. Appl. Phys. 32, 510 (1961) [CrossRef] [Google Scholar]
- L. Choubrac, A. Lafond, C. Guillot-Deudon, Y. Moëlo, S. Jobic, Inorg. Chem. 51, 3346 (2012) [CrossRef] [PubMed] [Google Scholar]
- USGS, Commodity Statistics and Information, USGS Minerals Information (2010), http://minerals.usgs.gov/minerals/pubs/commodity/ [Google Scholar]
- S. Bag, O. Gunawan, T. Gokmen, Y. Zhu, T.K. Todorov, D.B. Mitzi, Energy Environ. Sci. 5, 7060 (2012) [CrossRef] [Google Scholar]
- J.B. Li, V. Chawla, B.M. Clemens, Adv. Mat. 24, 720 (2012) [CrossRef] [Google Scholar]
- S. Siebentritt, S. Schorr, Prog. Photovolt. Res. Appl. in press [Google Scholar]
- S. Chen, X.G. Gong, A. Walsh, S.H. Wei, Phys. Rev. B 79, 165211 (2009) [CrossRef] [Google Scholar]
- S.R. Hall, J.T. Szymanski, J.M. Stewart, Can. Mineral. 16, 131 (1978) [Google Scholar]
- L.O. Brockway, Z. Kristaloogr. 89, 434 (1934) [Google Scholar]
- P. Bonazzi, L. Bindi, G.P. Bernardini, S. Menchetti, Can. Mineral. 41, 639 (2003) [CrossRef] [Google Scholar]
- S. Schorr, H.J. Hoebler, M. Tovar, Eur. J. Min. 19, 65 (2007) [CrossRef] [Google Scholar]
- S. Schorr, Sol. Energy Mater. Solar Cells 95, 1482 (2011) [CrossRef] [Google Scholar]
- J. Paier, R. Asahi, A. Nagoya, G. Kresse, Phys. Rev. B 79, 115126 (2009) [CrossRef] [Google Scholar]
- A. Walsh, S. Chen, X.G. Gong, S.H. Wei (CZTS) : Theoretical insights, http://web.mac.com/aronwalsh/publications/10/icps_czts_10.pdf [Google Scholar]
- A. Walsh, S.H. Wei, S. Chen, X.G. Gong, Design of quaternary chalcogenide photovoltaic absorbers through cation mutation, in 34th IEEE Photovoltaic Specialists Conference (2009) [Google Scholar]
- A. Walsh, S. Chen, S. Wei, X. Gong, Kesterite Thin-Film Solar Cells : Advances in Materials Modelling of Cu2ZnSnS4, Adv. Energy Mater. 2, 400 (2012) [Google Scholar]
- Y. Zhang, X. Sun, P. Zhang, X. Yuan, F. Huang, W. Zhang, J. Appl. Phys. 111, 063709 (2012) [CrossRef] [Google Scholar]
- H. Nozaki, T. Fukano, S. Ohta, Y. Seno, H. Katagiri, K. Jimbo, J. Alloys Compd. 524, 22 (2012) [CrossRef] [Google Scholar]
- X. He, H. Shen, Physica B : Condensed Matter 406, 4604 (2011) [CrossRef] [Google Scholar]
- X. He, H. Shen, Phys. Scr. 85, 035302 (2012) [CrossRef] [Google Scholar]
- P.A. Fernandes, P.M.P. Salomé, A.F. da Cunha, Thin Solid Films, 517, 2519 (2009) [CrossRef] [Google Scholar]
- J. Just, D. Lützenkirchen-Hecht, R. Frahm, S. Schorr, T. Unold, Appl. Phys. Lett. 99, 262105 (2011) [CrossRef] [Google Scholar]
- A. Nagoya, R. Asahi, R. Wahl, G. Kresse, Phys. Rev. B 81, 113202 (2010) [CrossRef] [Google Scholar]
- S. Chen, X.G. Gong, A. Walsh, S.H. Wei, Appl. Phys. Lett. 96, 021902 (2010) [CrossRef] [Google Scholar]
- P.J. Dale, K. Hoenes, J. Scragg, S. Siebentritt, A review of the challenges facing kësterite based thin film solar cells, in Photovoltaic Specialists Conference (PVSC), 2009 34th IEEE (2010), pp. 002080–002085 [Google Scholar]
- T. Maeda, S. Nakamura, T. Wada, Thin Solid Films 519, 7513 (2011) [CrossRef] [Google Scholar]
- M.J. Romero, H. Du, G. Teeter, Y. Yan, M.M. Al-Jassim, Phys. Rev. B 84, 165324 (2011) [CrossRef] [Google Scholar]
- E. Kask, T. Raadik, M. Grossberg, R. Josepson, J. Krustok, Energy Procedia 10, 261 (2011) [CrossRef] [Google Scholar]
- S. Chen, J.H. Yang, X.G. Gong, A. Walsh, S.H. Wei, Phys. Rev. B 81, 245204 (2010) [CrossRef] [Google Scholar]
- T. Maeda, S. Nakamura, T. Wada, Jpn J. Appl. Phys. 50, 04DP07 (2011) [CrossRef] [Google Scholar]
- A. Jeong, W. Jo, S. Jung, J. Gwak, J. Yun, Appl. Phys. Lett. 99, 082103 (2011) [CrossRef] [Google Scholar]
- A. Nagaoka, K. Yoshino, H. Taniguchi, T. Taniyama, H. Miyake, J. Cryst. Growth 341, 38 (2012) [CrossRef] [Google Scholar]
- T. Washio, H. Nozaki, T. Fukano, T. Motohiro, K. Jimbo, H. Katagiri, J. Appl. Phys. 110, 074511 (2011) [CrossRef] [Google Scholar]
- I. Repins et al., Kesterites and Chalcopyrites : A Comparison of Close Cousins, in MRS Proceedings (2011), Vol. 1324 [Google Scholar]
- K. Muska, M. Kauk, M. Altosaar, M. Pilvet, M. Grossberg, O. Volobujeva, Energy Procedia 10, 203 (2011) [CrossRef] [Google Scholar]
- H. Katagiri, K. Jimbo, M. Tahara, H. Araki, K. Oishi, The influence of the composition ratio on CZTS-based thin film solar cells, in Materials Research Society Symposium Proceedings (2009), p. 1165 [Google Scholar]
- A. Ennaoui, M. Lux-Steiner, A. Weber, D. Abou-Ras, I. Kötschau, H.-W. Schock, R. Schurr, A. Hölzing, S. Jost, R. Hock, T. Voß, J. Schulze, A. Kirbs, Thin Solid Films 517, 2511 (2009) [CrossRef] [Google Scholar]
- P.A. Fernandes, P.M.P. Salomé, A.F. da Cunha, Semicond. Sci. Technol. 24, 105013 (2009) [CrossRef] [Google Scholar]
- T.K. Todorov, K.B. Reuter, D.B. Mitzi, Adv. Mat. 22, (2010) [Google Scholar]
- K. Jimbo, R. Kimura, T. Kamimura, S. Yamada, W.S. Maw, H. Araki, K. Oishi, H. Katagiri, Thin Solid Films 515, 5997 (2007) [CrossRef] [Google Scholar]
- M. Valentini, C. Malerba, F. Biccari, R. Chierchia, P. Mangiapane, E. Salza, A. Mittiga, Growth and characterization of Cu2ZnSnS4 thin films prepared by sulfurization of evaporated precursors, in 26th EUPVSEC (Hamburg, 2011) [Google Scholar]
- S.J. Ahn, S. Jung, J. Gwak, A. Cho, K. Shin, K. Yoon, D. Park, H. Cheong, J.H. Yun, Appl. Phys. Lett. 021905 (2010) [Google Scholar]
- D. Park, D. Nam, S. Jung, S.J. An, J. Gwak, K. Yoon, J.H. Yun, H. Cheong, Thin Solid Films 519, 7386 (2011) [CrossRef] [Google Scholar]
- H. Wang, Int. J. Photoenergy 2011 (2011) [Google Scholar]
- M. Edoff, S. Schleussner, E. Wallin, O. Lundberg, Thin Solid Films 519, 7530 (2011) [CrossRef] [Google Scholar]
- S. Ahmed, K.B. Reuter, O. Gunawan, L. Guo, L.T. Romankiw, H. Deligianni, Adv. Energy Mater. in press [Google Scholar]
- B. Shin, O. Gunawan, Y. Zhu, N.A. Bojarczuk, S.J. Chey, S. Guha, Prog. Photovolt. Res. Appl. in press [Google Scholar]
- I. Repins, C. Beall, N. Vora, C. DeHart, D. Kuciauskas, P. Dippo, B. To, J. Mann, W.-C. Hsu, A. Goodrich, R. Noufi, Solar Energy Mater. Solar Cells, in press [Google Scholar]
- M. Kauk, K. Muska, M. Altosaar, J. Raudoja, M. Pilvet, T. Varema, K. Timmo, O. Volobujeva, Energy Procedia 10, 197 (2011) [CrossRef] [Google Scholar]
- B.-A. Schubert, B. Marsen, S. Cinque, T. Unold, R. Klenk, S. Schorr, H.-W. Schock, Prog. Photovolt Res. Appl. 19, 93 (2011) [CrossRef] [Google Scholar]
- E. Mellikov, D. Meissner, T. Varema, M. Altosaar, M. Kauk, O. Volobujeva, J. Raudoja, K. Timmo, M. Danilson, Energy Mater. Solar Cells 93, 65 (2009) [CrossRef] [Google Scholar]
- X.Y. Li, D.C. Wang, Q.Y. Du, W.F. Liu, G.S. Jiang, C.F. Zhu, Adv. Mater. Res. 418-420, 67 (2011) [CrossRef] [Google Scholar]
- T.K. Chaudhuri, D. Tiwari, Solar Energy Mater. Solar Cells 101, 46 (2012) [CrossRef] [Google Scholar]
- T. Rath, W. Haas, A. Pein, R. Saf, E. Maier, B. Kunert, F. Hofer, R. Resel, G. Trimmel, Solar Energy Mater. Solar Cells 101, 87 (2012) [CrossRef] [Google Scholar]
- N.M. Shinde, C.D. Lokhande, J.H. Kim, J.H. Moon, J. Photochem. Photobiol. A : Chem., in press [Google Scholar]
- R.A. Wibowo, W.S. Kim, E.S. Lee, B. Munir, K.H. Kim, J. Phys. Chem. Solids 68, 1908 (2007) [CrossRef] [Google Scholar]
- F. Hergert, R. Hock, Thin solid films 515, 5953 (2007) [CrossRef] [Google Scholar]
- A.J. Cheng, M. Manno, A. Khare, C. Leighton, S. Campbell, E. Aydil, J. Vac. Sci. Technol. A Vac. Surf. Films 29, 051203 (2011) [CrossRef] [Google Scholar]
- O. Volobujeva, J. Raudoja, E. Mellikov, M. Grossberg, S. Bereznev, R. Traksmaa, J. Phys. Chem. Solids 70, 567 (2009) [CrossRef] [Google Scholar]
- R. Schurr et al., Thin Solid Films 517, 2465 (2009) [CrossRef] [Google Scholar]
- M. Ganchev, J. Iljina, L. Kaupmees, T. Raadik, O. Volobujeva, A. Mere, M. Altosaar, J. Raudoja, E. Mellikov, Thin Solid Films 519, 7394 (2011) [CrossRef] [Google Scholar]
- K. Maeda, K. Tanaka, Y. Nakano, H. Uchiki, Jpn J. Appl. Phys. 50, 05FB08 (2011) [CrossRef] [Google Scholar]
- A. Redinger, S. Siebentritt, Appl. Phys. Lett. 97, 092111 (2010) [CrossRef] [Google Scholar]
- H. Yoo, J. Kim, Thin Solid Films 518, 6567 (2010) [CrossRef] [Google Scholar]
- J. Ge, Y. Wu, C. Zhang, S. Zuo, J. Jiang, J. Ma, P. Yang, J. Chu, Appl. Surf. Sci. 258, 7250 (2012) [CrossRef] [Google Scholar]
- K. Maeda, K. Tanaka, Y. Fukui, H. Uchiki, Solar Energy Mater. Solar Cells 95, 2855 (2011) [CrossRef] [Google Scholar]
- K. Maeda, K. Tanaka, Y. Nakano, Y. Fukui, H. Uchiki, Jpn J. Appl. Phys. 50, 05FB09 (2011) [CrossRef] [Google Scholar]
- J.J. Scragg, Studies of Cu2ZnSnS4 Films Prepared by Sulfurisation of Electrodeposited Precursors, Ph.D. thesis, University of Bath, Department of Chemistry, Bath, 2010 [Google Scholar]
- G. Teeter, H. Du, J.E. Leisch, M. Young, F. Yan, S. Johnston, P. Dippo, D. Kuciauskas, M. Romero, P. Newhouse, S. Asher, D. Ginley, Combinatorial study of thin-film Cu2ZnSnS4 synthesis via metal precursor sulfurization, in 35th IEEE-PVSEC (2010) [Google Scholar]
- A. Weber, R. Mainz, H.W. Schock, J. Appl. Phys. 107, 013516 (2010) [CrossRef] [Google Scholar]
- H. Yoo, J. Kim, L. Zhang, Curr. Appl. Phys. in press [Google Scholar]
- A. Redinger, D.M. Berg, P.J. Dale, R. Djemour, L. Gutay, T. Eisenbarth, N. Valle, S. Siebentritt, IEEE J. Photovoltaics 1, 200 (2011) [CrossRef] [Google Scholar]
- A. Redinger, D.M. Berg, P.J. Dale, S. Siebentritt, J. Am. Chem. Soc. 156 (2011) [Google Scholar]
- C.M. Fella, G. Ilari, A.R. Uhl, A. Chirilă, Y.E. Romanyuk, A.N. Tiwari, Non-vacuum deposition of Cu2ZnSnSe4 absorber layers for thin film solar cells, in 26 EUPVSEC (Hamburg, 2011) [Google Scholar]
- F. Biccari, R. Chierchia, M. Valentini, P. Mangiapane, E. Salza, C. Malerba, C.L.A. Ricardo, L. Mannarino, P. Scardi, A. Mittiga, Energy Procedia 10, 187 (2011) [CrossRef] [Google Scholar]
- S. Guha, D.B. Mitzi, T.K. Todorov, K. Wang, Annealing Thin Films, US Patent United States Patent Application 2012007093622, 2012 [Google Scholar]
- P. Salomé, J. Malaquias, P. Fernandes, M. Ferreira, J. Leitão, A. da Cunha, J. González, F. Matinaga, G. Ribeiro, E. Viana, Solar Energy Mater. Solar Cells 95, 3482 (2011) [CrossRef] [Google Scholar]
- K. Wang, B. Shin, K.B. Reuter, T. Todorov, D.B. Mitzi, S. Guha, Appl. Phys. Lett. 98, 051912 (2011) [CrossRef] [Google Scholar]
- R. Juškėnas, S. Kanapeckaitė, V. Karpavičienė, Z. Mockus, V. Pakštas, A. Selskienė, R. Giraitis, G. Niaura, Solar Energy Mater. Solar Cells 101, 277 (2012) [CrossRef] [Google Scholar]
- S.W. Shin, S. Pawar, C.Y. Park, J.H. Yun, J.H. Moon, J.H. Kim, J.Y. Lee, Solar Energy Mater. Solar Cells 95, 3202 (2011) [CrossRef] [Google Scholar]
- L. Grenet, S. Bernardi, D. Kohen, C. Lepoittevin, S. Noël, N. Karst, A. Brioude, S. Perraud, H. Mariette, Solar Energy Mater. Solar Cells 101, 11 (2012) [CrossRef] [Google Scholar]
- H. Yoo, J. Kim, Solar Energy Mater. Solar Cells 95, 239 (2011) [CrossRef] [Google Scholar]
- R. Chalapathy, G.S. Jung, B.T. Ahn, Solar Energy Mater. Solar Cells 95, 3216 (2011) [CrossRef] [Google Scholar]
- W. Hlaing Oo, J. Johnson, A. Bhatia, E. Lund, M. Nowell, M. Scarpulla, Journal of Electronic Materials 40, 2214 [Google Scholar]
- T. Prabhakar, N. Jampana, Solar Energy Mater. Solar Cells 95, 1001 (2011) [CrossRef] [Google Scholar]
- M. Bär, B.A. Schubert, B. Marsen, S. Krause, S. Pookpanratana, T. Unold, L. Weinhardt, C. Heske, H.W. Schock, Appl. Phys. Lett. 99, 112103 (2011) [CrossRef] [Google Scholar]
- A. Nagoya, R. Asahi, G. Kresse, J. Phys. Condens. Matter 23, 404203 (2011) [CrossRef] [PubMed] [Google Scholar]
- N. Sakai, H. Hiroi, H. Sugimoto, Development of cd-free buffer layer for Cu2ZnSnS4 thin-film solar cells, in 37th IEEE PVSC Conference (2011) [Google Scholar]
- F. Jiang, H. Shen, W. Wang, L. Zhang, Appl. Phys. Expr. 4, 074101 (2011) [CrossRef] [Google Scholar]
- P.K. Sarswat, M.L. Free, Phys. Status Solidi 208, 2861 (2011) [CrossRef] [Google Scholar]
- P.K. Sarswat, M. Snure, M.L. Free, A. Tiwari, Thin Solid Films 520, 1694 (2012) [CrossRef] [Google Scholar]
- Q.-M. Chen, Z.-Q. Li, Y. Ni, S.-Y. Cheng, X.-M. Dou, Chin. Phys. B 21, 038401 (2012) [CrossRef] [Google Scholar]
- Q. Tian, X. Xu, L. Han, M. Tang, R. Zou, Z. Chen, M. Yu, J. Yang, J. Hu, Cryst. Eng. Commun., 14, 3847 (2012) [CrossRef] [Google Scholar]
- O. Gunawan, T.K. Todorov, D.B. Mitzi, Appl. Phys. Lett. 97, 233506 (2010) [CrossRef] [Google Scholar]
- K. Wang, O. Gunawan, T. Todorov, B. Shin, S.J. Chiy, N.A. Bojarczuk, D. Mitzi, S. Guha, Appl. Phys. Lett. 97, 143508 (2010) [CrossRef] [Google Scholar]
- B. Shin, K. Wang, O. Gunawan, K.B. Reuter, S.J. Chey, N.A. Bojarczuk, T. Todorov, B. Mitzi, S. Guha, in 37th IEEE PVSC Conference (Seattle, 2011), Vol. 1 [Google Scholar]
- M. Bär, B.-A. Schubert, B. Marsen, R.G. Wilks, M. Blum, S. Krause, S. Pookpanratana, Y. Zhang, T. Unold, W. Yang, L. Weinhardt, C. Heske, H.-W. Schock, J. Mater. Res. FirstView, 1 [Google Scholar]
- T. Tanaka, T. Sueishi, K. Saito, Q. Guo, M. Nishio, K.M. Yu, W. Walukiewicz, J. Appl. Phys. 111, 053522 (2012) [CrossRef] [Google Scholar]
- M. Bär, B.-A. Schubert, B. Marsen, S. Krause, S. Pookpanratana, T. Unold, L. Weinhardt, C. Heske, H.-W. Schock, Appl. Phys. Lett. 99, 152111 (2011) [CrossRef] [Google Scholar]
- M. Bär, B.-A. Schubert, B. Marsen, R.G. Wilks, S. Pookpanratana, M. Blum, S. Krause, T. Unold, W. Yang, L. Weinhardt, C. Heske, H.-W. Schock, Appl. Phys. Lett. 99, 222105 (2011) [CrossRef] [Google Scholar]
- R. Haight, A. Barkhouse, O. Gunawan, B. Shin, M. Copel, M. Hopstaken, D.B. Mitzi, Appl. Phys. Lett. 98, 253502 (2011) [CrossRef] [Google Scholar]
- J.M. Raulot, C. Domain, J.F. Guillemoles, J. Phys. Chem. Solids 66, 2019 (2005) [CrossRef] [Google Scholar]
- A. Opanasyuk, D. Kurbatov, M. Ivashchenko, I.Y. Protsenko, H. Cheong, Journal of Nano- and Electronic Physics 4, 01024 (2012) [Google Scholar]
- C. Tao, J. Jiang, M. Tao, ECS Transactions 33, 3 (2011) [CrossRef] [Google Scholar]
- D.B. Mitzi, T.K. Todorov, Method of Forming Semiconductor Film and Photovoltaic Device Including the Film, US Patent US2011094557 (A1)Apr-2011 [Google Scholar]
- D.B. Mitzi, T.K. Todorov, Aqueous-based method of forming semiconductor film and photovoltaic device including the film, US Patent US 2011097496 (A1), 2011 [Google Scholar]
- E. Mellikov, M. Altosaar, J. Raudoja, K. Timmo, O. Volobujeva, M. Kauk, J. Krustok, T. Varema, M. Grossberg, M. Danilson, K. Muska, K. Ernits, F. Lehner, D. Meissner, Materials Challenges in Alternative and Renewable Energy : Ceramic Transactions 224, 137 [Google Scholar]
- J.J. Scragg, P.J. Dale, L.M. Peter, Electrochem. Commun. 10, 639 (2008) [CrossRef] [Google Scholar]
- J.J. Scragg, D.M. Berg, P.J. Dale, J. Electroanal. Chem. 646, 52 (2010) [CrossRef] [Google Scholar]
- H. Araki, Y. Kubo, A. Mikaduki, K. Jimbo, W.S. Maw, H. Katagiri, M. Yamazaki, K. Oishi, A. Takeuchi, Solar Energy Mater. Solar Cells 93, 996 (2009) [CrossRef] [Google Scholar]
- J.J. Scragg, P.J. Dale, L.M. Peter, Thin Solid Films 517, 2481 (2009) [CrossRef] [Google Scholar]
- J.J. Scragg, P.J. Dale, L.M. Peter, G. Zoppi, I. Forbes, Phys. Stat. Sol. B 245, 1772 (2008) [CrossRef] [Google Scholar]
- M. Kurihara, D. Berg, J. Fischer, S. Siebentritt, P.J. Dale, Physica Status Solidi 6, 1241 (2009) [CrossRef] [Google Scholar]
- X. Zhang, X. Shi, W. Ye, C. Ma, C. Wang, Appl. Phys. A 94, 381 (2009) [CrossRef] [Google Scholar]
- H. Deligianni, L. Guo, R. Vaidyanathan, Electrodeposition of Thin-Film Cells Containing Non-Toxic Elements, US Patent United States Patent Application 2012004837803, 2012 [Google Scholar]
- S. Ahmed, H. Deligianni, L.T. Romankiw, K. Wang, Structure and Method of Fabricating a CZTS Photovoltaic Device by Electrodeposition, US Patent United States Patent Application 2012006179015, 2012 [Google Scholar]
- Q. Guo, G.M. Ford, W.C. Yang, B.C. Walker, E.A. Stach, H.W. Hillhouse, R. Agrawal, J. Am. Chem. Soc. 132, 2844 (2010) [Google Scholar]
- T. Washio, T. Shinji, S. Tajima, T. Fukano, T. Motohiro, K. Jimbo, H. Katagiri, J. Mater. Chem., 22, 4021 (2012) [CrossRef] [Google Scholar]
- K. Woo, Y. Kim, J. Moon, Energy Environ. Sci. 5, 5340 (2012) [CrossRef] [Google Scholar]
- W. Ki, H.W. Hillhouse, Adv. Energy Mater. 1, 732 (2011) [CrossRef] [Google Scholar]
- N. Momose, M.T. Htay, T. Yudasaka, S. Igarashi, T. Seki, S. Iwano, Y. Hashimoto, K. Ito, Jpn. J. Appl. Phys. 50, 01BG09 (2011) [CrossRef] [Google Scholar]
- H. Flammersberger, Experimental study of Cu2ZnSnS4 thin films for solar cells, Master thesis, Uppsala University, 2010 [Google Scholar]
- C. Platzer-Björkman, J. Scragg, H. Flammersberger, T. Kubart, M. Edoff, Solar Energy Mater. Solar Cells 98, 110 (2012) [CrossRef] [Google Scholar]
- K.H. Kim, I. Amal, Electronic Materials Letters 7, 225 (2011) [CrossRef] [Google Scholar]
- H. Araki, Y. Kubo, K. Jimbo, W.S. Maw, H. Katagiri, M. Yamazaki, K. Oishi, A. Takeuchi, Phys. Status Solidi 6, 1266 (2009) [CrossRef] [Google Scholar]
- H. Kühnlein, J. Schulze, T. Voss, Metal Plating Composition And Method For The Deposition Of Copper-zinc-tin Suitable For Manufacturing Thin Film Solar Cell, US Patent US2009/0205714 (A1)20, 2009 [Google Scholar]
- H.H. Kühnlein, Elektrochemische Legierungsabscheidung zur Herstellung von Cu2ZnSnS4 Dünnschichtsolarzellen, Ph.D. thesis, Dresden, 2007 [Google Scholar]
- M.L. Free, P.K. Sarswat, A. Tiwari, M. Snure, Modified copper-zinc-tin semiconductor films, uses thereof and related methods, US Patent United States Patent Application 20110132462A106, 2011 [Google Scholar]
- B.S. Pawar, S.M. Pawar, S.W. Shin, D.S. Choi, C.J. Park, S.S. Kolekar, J.H. Kim, Appl. Surf. Sci. 257, 1786 (2010) [CrossRef] [Google Scholar]
- S.M. Pawar, B.S. Pawar, A.V. Moholkar, D.S. Choi, J.H. Yun, J.H. Moon, S.S. Kolekar, J.H. Kim, Electrochimica Acta 55, 4057 (2010) [CrossRef] [Google Scholar]
- M. Jeon, Y. Tanaka, T. Shimizu, S. Shingubara, Energy Procedia 10, 255 (2011) [CrossRef] [Google Scholar]
- M. Jeon, T. Shimizu, S. Shingubara, Mater. Lett. 65, 2364 (2011) [CrossRef] [Google Scholar]
- G. Zoppi, I. Forbes, R.W. Miles, P.J. Dale, J.J. Scragg, L.M. Peter, Progress in Photovoltaics : Research and Applications 17, 315 (2009) [CrossRef] [Google Scholar]
- K. Moriya, K. Tanaka, H. Uchiki, Jpn J. Appl. Phys. 46, 5780 (2007) [CrossRef] [Google Scholar]
- A.V. Moholkar, S.S. Shinde, A.R. Babar, K.-U. Sim, Y. Kwon, K.Y. Rajpure, P.S. Patil, C.H. Bhosale, J.H. Kim, Solar Energy 85, 1354 (2011) [CrossRef] [Google Scholar]
- L. Sun, J. He, H. Kong, F. Yue, P. Yang, J. Chu, Solar Energy Mater. Solar Cells 95, 2907 (2011) [CrossRef] [Google Scholar]
- A. Moholkar, S. Shinde, A. Babar, K. Sim, K. Hyun, K. Rajpure, P. Patil, C. Bhosale, J. Kim, J. Alloys Compd. 509, 7439 (2011) [CrossRef] [Google Scholar]
- K. Tanaka, Y. Fukui, N. Moritake, H. Uchiki, Solar Energy Mater. Solar Cells 95, 838 (2011) [CrossRef] [Google Scholar]
- C. Lokhande, N. Shinde, J. Kim, J. Moon, Invertis Journal of Renew. Energy 1, 142 (2011) [Google Scholar]
- S.S. Mali, B.M. Patil, C.A. Betty, P.N. Bhosale, Y.W. Oh, S.R. Jadkar, R.S. Devan, Y.-R. Ma, P.S. Patil, Electrochimica Acta 66, 216 (2012) [CrossRef] [Google Scholar]
- H. Araki, A. Mikaduki, Y. Kubo, T. Sato, K. Jimbo, W.S. Maw, H. Katagiri, M. Yamazaki, K. Oishi, A. Takeuchi, Thin Solid Films 517, 1457 (2008) [CrossRef] [Google Scholar]
- F. Liu, Y. Li, K. Zhang, B. Wang, C. Yan, Y. Lai, Z. Zhang, J. Li, Y. Liu, Solar Energy Mater. Solar Cells 94, 2431 (2010) [CrossRef] [Google Scholar]
- V. Chawla, B. Clemens, Inexpensive, abundant, non-toxic thin films for solar cell applications grown by reactive sputtering, in Photovoltaic Specialists Conference (PVSC), 2010 35th IEEE (2010), pp. 001902–001905 [Google Scholar]
- A. Wangperawong, J.S. King, S.M. Herron, B.P. Tran, K. Pangan-Okimoto, S.F. Bent, A chemical bath process for depositing Cu2ZnSnS4 photovoltaic absorbers, in 35th IEEE-PVSEC (Hawai, 2010) [Google Scholar]
- C. Shi, G. Shi, Z. Chen, P. Yang, M. Yao, Mater. Lett. 73, 89 (2012) [CrossRef] [Google Scholar]
All Tables
All Figures
 |
Fig. 1 Evolution of the record efficiency for CZTS solar cells. See Table 2 for the references. |
| In the text | |
 |
Fig. 2 Cu2ZnSn(S, Se)4 compound in kësterite structure, according to [33]. |
| In the text | |
 |
Fig. 3 Composition map of the best CZTS solar cells. The diameter of the circles is proportional to the conversion efficiency. See Table 2 for the references. |
| In the text | |
 |
Fig. 4 SEM cross-section of CZTSe films grown at the same temperature and constant deposition fluxes, except Cu rates were adjusted to produce only (a) Cu-rich growth or (b) Cu-poor growth [59]. |
| In the text | |
 |
Fig. 5 Classical architecture for CZTS solar cells. |
| In the text | |
 |
Fig. 6 CZTSe with ZnSe segregation at the back contact [59]. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


